Search This Blog
Categories
Most Popular

SOP for Serious Adverse Events (SAE) Reconciliation
December 12, 2024

Comprehensive Guide to Pharmacovigilance
January 06, 2025

The Role of Artificial Intelligence in Modern Pharmacovigilance
November 30, 2024

Understanding PADER: Periodic Adverse Drug Experience Report
November 30, 2024
.webp)
Pharmacovigilance System Master File (PSMF)
December 12, 2024

Guide to Developing Effective Pharmacovigilance SOPs
December 01, 2024

SOP for Signal Management
January 13, 2025

SOP for Handling and reporting of AE and SAE
September 07, 2025
Featured post
SOP for Handling and reporting of AE and SAE
Mubarak Patel-
September 07, 2025
SOP for the Study Master File
Mubarak Patel
May 14, 2025
Purpose
To describe the procedures related to the maintenance of the Study Master File (SMF) held at all clinical research sites/units, according to ICH GCP E6 (R2) Section 8 to ensure it is current at all times for the duration of the clinical study.
Scope
This SOP applies to all relevant employees, including but not limited to, visiting health professionals, contractors, consultants and volunteers who propose to undertake, administrate, review and/or govern human research involving patients/participants, facilities and or staff. All study personnel involved in the clinical study must operate within their scope of practice.
Procedure
The Study Master File – Principal Investigator Responsibilities
- The Principal Investigator must:
- Ensure an SMF is created, if not provided by the Sponsor, prior to study commencement and ensure that it contains at a minimum the Essential Documents listed in Annexure-1 Study Master File Index Example. The SMF is stored at the Primary Site (Satellite Site Study File in the case of the PI initiating a Satellite Site).
- Where the Teletrial Model is implemented, have control of all Essential Documents and records generated by the Investigator/Institution/Satellite Site before, during, and after the trial.
- Establish the maintenance rules of the SMF and relationship between the Primary Site Study Master File (SMF) and Satellite Site Study File (SSSF). For example, the contents of the SSSF, how and which documents generated at the Satellite Site will be sent to the Primary Site and filed in the SMF and archiving of Satellite Site Study File after study close out. When establishing the maintenance rules, it will be important to ensure that key documents from the SSSF are present in the SMF and vice-versa after the close out of the study but prior to archiving, so that a full record of all study activities under the control of the Principal Investigator (PI) is contained in the SMF. As the SMF contains identifiable data and proprietary information, it should also be stored securely with restricted access to authorised staff.
- Establish prior to the commencement of the trial and maintain a current record of the location of all Essential Documents including Source Documents and where relevant, study related Essential Documents from the Satellite Site. The storage system used during the study and for archiving (irrespective of the type of media used) should provide for document identification and location, version history, search-ability and retrieval for the length of the archiving retention time.
- File Essential Documents in a timely manner.
- Ensure Satellite Sites also maintain SSSF and file study related Essential Documents in a timely manner, with focus on version control.
- Maintain a current contact list of all Study Personnel including staff at all Satellite Site/s within the Cluster involved in the clinical trial, clearly identifying the Primary Site, the Satellite Site and any external service provider.
- Ensure study documentation is kept and archived as specified in Site Close-Out and Archiving.
ALSO READ: Pharmacovigilance System Master File (PSMF)
The Study Master File
- Study related Essential and Source Documents generated for/by the Primary Site, as per Annexure-1 at a minimum, will be filed in the SMF.
- The Study Master File (SMF) should be prefaced with an index of contents as well as indicate the location(s) of all Essential/Source Documents.
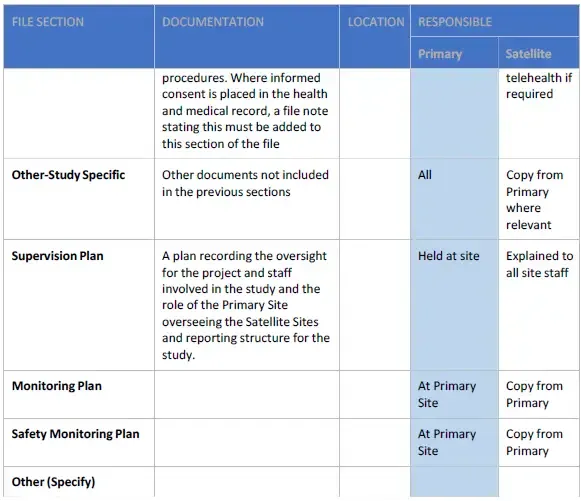
- Certified copies of study related Essential and Source Documents generated for/by the Satellite Site, the identity of which will be established prior to the commencement of the trial, will be sent to the Primary Site and filed in the SMF, on request by either, the Sponsor, monitor or Primary Site staff as per rules established prior to the commencement of the trial and documented in the Supervision Plan.
- Where financial documentation, such as the Clinical Trial Agreement and Sub-Contract, invoicing and remittances etc. may be filed in a separate location to the SMF, the location is to be recorded on the SMF index. A copy may be filed in the SMF if requested by the Sponsor.
- Investigational Product handling documentation e.g. shipping, receipt, Interactive Voice Response System (IVRS), Interactive Web Response System (IWRS), codes, randomisation list and accountability and destruction documents etc. may be kept in a separate file e.g. at the site pharmacy. In this case the location is to be recorded on the SMF index. However, the records must be made available to Sponsors, monitors, auditors and regulatory agencies at any time.
- The Investigational Product documentation will be archived with the SMF after completion of the study.
- Sample handling procedures are to be clearly documented if performed e.g. in a laboratory manual. Sample management records at both Primary and Satellite Site/s including the storage, processing and transportation of samples between Satellite and Primary Sites are filed in the SMF/SSSF as agreed.
- Other study related materials handling documentation are filed in the SMF/SSSF as agreed.
The Study Master File – Contents
The content of the Satellite Site Study File (SSSF) can be decided with the study team and the Sponsor. The SSSF may be a sub-set of the Study Master File (SMF) and should be prefaced with an index of contents as well as indicate the location(s) of all Essential/Source Documents. The Satellite Site Study File should contain:
- All the relevant site-specific Essential Documentation pertinent to the activities that have been and that are to be performed at the Satellite Site, similar to Appendix-1.
- All Source Documents generated at the Satellite Site (or indicate the location of all Source Documents for example the EMR at the Satellite Site).
- Relevant HREC approval and governance authorisation documentation.
- Sub-Contract with the Clinical Trial Agreement in annexure.
- Study specific Supervision Plan.
- Satellite Site Delegation Log.
- Satellite Site Training Records.
- Satellite Site, Site Specific Assessment form.
- Investigational Product shipping, receipt and accountability documents.
- Details of the processing, storage of samples at both Sites and transportation between Satellite and Primary Sites and related documentation (if performed).
- Files notes indicating if the original document is found in another location e.g. pharmacy folder with the pharmacy, a document will be found in the SMF.
Annexure
Translate
Popular Posts
Pharmacovigilance System Master File (PSMF)
December 12, 2024
SOP for Serious Adverse Events (SAE) Reconciliation
December 12, 2024
SOP for Pharmacovigilance
November 08, 2024
Understanding PADER: Periodic Adverse Drug Experience Report
November 30, 2024
SOP for Signal Detection and Risk Management
January 02, 2025
SOP for Protocol and Investigational Brochure Requirements
April 29, 2025
Oral Cholera Vaccine
February 05, 2025
About

Pharmacovigilance is an educational blog dedicated to providing clear, accessible, and well-structured insights into the fields of pharmacovigilance and vaccine manufacturing. Designed for both industry professionals and students, the blog simplifies complex concepts to enhance understanding and practical application. Contact us at Email: admin@pharmacovigilances.com
Popular Posts
SOP for Serious Adverse Events (SAE) Reconciliation
December 12, 2024
Pharmacovigilance System Master File (PSMF)
December 12, 2024
Footer Menu Widget
Copyright © 2025. Pharmacovigilance | All Right Reserved.
Created By Blogspot Theme | Distributed By Gooyaabi Templates






0 Comments